
Work Bridgeable
Bridging the Gap: Expanding access to clinical trials in New Brunswick
Problem
Horizon Health Network sought to improve access to clinical trials across New Brunswick for residents who experience difficulties in accessing hospitals or research sites where trial activities typically occur.
Solution
We engaged with potential Decentralized Clinical Trial (DCT) participants and researchers through interviews and workshops and delivered key principles, features for a digital platform, and a future delivery pathway to guide next steps.
Impact
This project helped our client to understand the current state of DCTs, including the needs of potential trial participants, caregivers and researchers. It continues to empower Horizon to make informed decisions as they align around a unified vision for the ideal DCT delivery experience.
Author
- Bridgeable
Client
- Horizon Health Network
The changing landscape of healthcare delivery
Since the COVID-19 pandemic, many Canadian healthcare organizations (HCOs) have expanded the delivery of virtual and community-based health services to address patient accessibility. Increased digital literacy in the general population, evolving patient expectations, and the ubiquity of video conferencing tools and personal devices have made tele- and e-health services a feasible way to increase service access for patients. At the same time, HCOs have come to recognize the value of providing essential health services in medical and non-medical community settings1,2, as seen in the distribution of COVID-19 vaccines through local pharmacies and pop-up sites. For many patients, the option of receiving basic health services in their community can significantly increase their access to care.
Bringing research closer to people: Decentralized clinical trials
This focus on access is transforming the design of clinical trials. Increasingly, investigators are using health technologies to connect with trial participants and capture data. This approach, in which some or all components of a trial are conducted remotely, is called a decentralized clinical trial (DCT). Although relatively new, there is a growing understanding that DCTs have the potential to increase patient representation by making research more accessible for groups who have traditionally been excluded.
Traditional clinical trials require patients to visit a designated trial site for a baseline assessment and informed consent, followed by subsequent visits for ongoing data collection, drug administration, or other necessary procedures. This in-person requirement introduces access barriers for patients who are unable to travel due to distance or time constraints, caregiving, family or work responsibilities, disability, or other challenges. In regions with large rural populations, such as New Brunswick (NB) and the other Atlantic provinces, patients must relocate in order to participate or be forced to decline, effectively excluding them from health innovation.
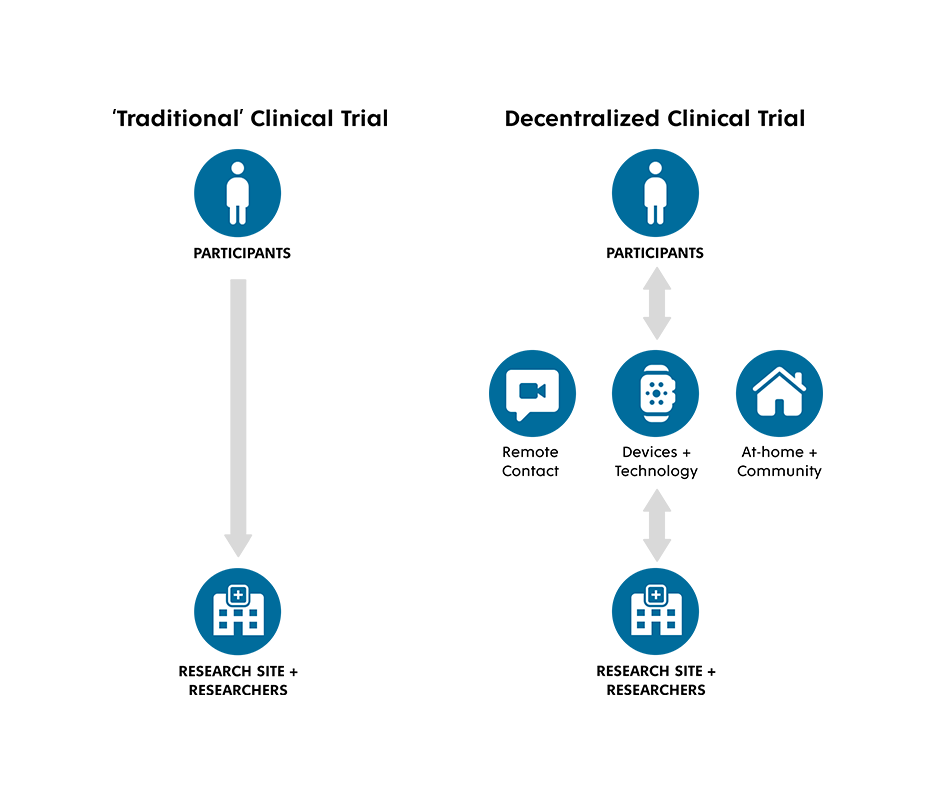
In contrast, investigators in DCTs may use video calls, digital surveys, or remote monitoring devices like smartwatches and other types of medical monitoring equipment capable of capturing and transmitting data remotely. By removing the in-person requirement, DCTs have the potential to drastically widen access for people who experience challenges that impact their ability to travel to research facilities.
In NB, where half of residents live in remote or rural communities, geographic and seasonal barriers (i.e. inclement weather) result in challenges with recruiting and maintaining participant engagement. Additionally, these barriers can amplify inequalities experienced by individuals from historically marginalized and underserved communities. As one of two health authorities in NB, Horizon Health Network (Horizon) oversees the delivery of healthcare services to half a million people and supports around 500 research studies a year. Horizon has an ambitious objective to address these barriers by creating a digital platform to support the delivery of DCTs and modernize clinical trial delivery in the province.
A human-centered approach to understanding participant and researcher needs
Clinical trials require willing and engaged participants, caregivers, and researchers, all of whom have conversations about the trial, ask questions or provide explanations, follow directives set out in the trial protocol, and collect or report data appropriately and conscientiously. Such human engagement is a cornerstone of the clinical trial and is an essential consideration for the usability of a DCT delivery platform. Therefore, understanding the needs of future platform users, such as trial participants and researchers in New Brunswick, is foundational to developing an accessible and relevant DCT platform.
Horizon and Bridgeable partnered to understand these needs by engaging stakeholders through workshops and interviews to uncover:
- Participant and caregiver attitudes, challenges, and needs related to participating in DCTs,
- Service gaps and opportunities for innovation within existing remote data capture technologies and,
- The current experience of designing and delivering DCTs.
We used a four-stage, collaborative co-design process to articulate, visualize, and validate a future-state DCT platform pathway and delivery features that support conversations, planning activities, and development.
Connected with
20
patients and researchers for interviews and workshops
Convened
15+
members of the New Brunswick research Community of Practice
Potential impact on
~500
research studies in New Brunswick
Stage 1: Capturing the current state of DCTs in New Brunswick
We began by completing an audit of clinical trials in New Brunswick to better understand the existing tools, processes, and challenges in trial delivery. We examined available digital tools and end-to-end platforms that aim to support DCT delivery to understand current features, functions, and opportunities for innovation.
We learned that:
- A DCT platform can benefit from patient familiarity with technologies such as video conferencing or remote data capture. Additionally, patient behavior that is becoming the norm across digital devices, such as logging personal health information, can be leveraged in the collection of trial data.
- Existing end-to-end DCT platforms, such as Medable or ObvioHealth, are differentiated by offering features that enable integration with EMRs, interoperability with personal devices, and by providing standardized tools for data collection and management.
- Decentralization adds complexity to the delivery of clinical trials. The ability to coordinate an ecosystem of trial activities and devices across multiple locations is a core value proposition for a DCT platform.
- The disparities that remote healthcare can exacerbate, such as through digital divides or lack of access to the internet and devices, means that technology alone cannot solve the problem of accessibility in research participation.
Stage 2: Uncovering the needs of DCT platform users
To understand the needs of potential DCT platform users, we convened two groups of stakeholders.
- Patients and Caregivers: We conducted two workshops with residents of New Brunswick who had an interest in participating in clinical trials, either as participants or caregivers. These workshops helped us understand attitudes, preferences, and concerns about participating in DCTs and allowed us to test potential scenarios and possible methods for supporting DCT delivery.
- Researchers in New Brunswick: We conducted interviews with researchers in New Brunswick who had experience running clinical trials with remote components, such as video calls, remote monitoring devices, or at-home visits. These interviews helped us understand the experience of researchers and provided insight into successes and challenges encountered when running trials with remote components.
What we heard
Through these interviews and workshops, we learned that maintaining participant engagement and minimizing the burden on researchers is crucial for designing and delivering a successful DCT.
Participants need to understand how they can contribute.
Participants are keen to take part in research studies to make a societal impact, but they need to know that they are contributing meaningfully.
We heard that the desire to contribute to the development of new treatments that may benefit future generations was a core motivator to participate in research.
“I would like to know that what I’ve done made a difference. I would like to know [the treatment] is an option, if it becomes relevant for me or a family member.” – Workshop Participant
For many participants, understanding the impact of their contributions, on both the large and small scale, can ‘make or break’ the experience of participating in trials. On the small scale, participants want reassurance that they are reporting data accurately. They also want to see the big picture, such as study outcomes and clinical impact. These motivations highlight the opportunity to reflect the contributions participants are making throughout a trial journey, and to more effectively engage participants before and after trials to educate and facilitate future recruitment.
Researchers are looking for ways to coordinate and integrate study processes to manage the complexity of running DCTs.
Researchers shared that there are multiple parts of the research process that can be time-consuming, redundant, and take significant resources to complete, such as aggregating multiple data points into a format that can be easily analyzed.
“Using multiple platforms for data management and capture is incredibly frustrating in many ways”
– Researcher
We learned that conducting remote data collection often involves coordinating with an ecosystem of external vendors and community partners, for example, couriers who pick up blood samples and deliver devices. Researchers need timely and responsive processes for integrating these digital and physical touchpoints across the trial journey.
Stage 3: Guiding DCT platform development: Bringing together critical stakeholders
Our next step was to convene a community of practice (CoP), including policy experts, researchers, administrators, and industry stakeholders who have an interest in conducting or supporting DCTs and clinical trials in New Brunswick. The CoP will support Horizon in future phases of this work to ensure industry buy-in, to de-risk development by incorporating key decision-makers early on, and to anticipate future uncertainties.
Stage 4: Bringing it all together
The insights from this work enabled us to develop three primary outputs to inform Horizon’s path forward. These outputs capture the relevant needs of potential DCT platform users and articulate the important experience moments for stakeholders.
1. Experience Principles
Capturing what matters most to participants and researchers, the Experience Principles are broad considerations for creating an ideal end-to-end platform experience. These principles are intended to guide development, motivate and align stakeholders, inform investment priorities, and evaluate the success of future innovation.
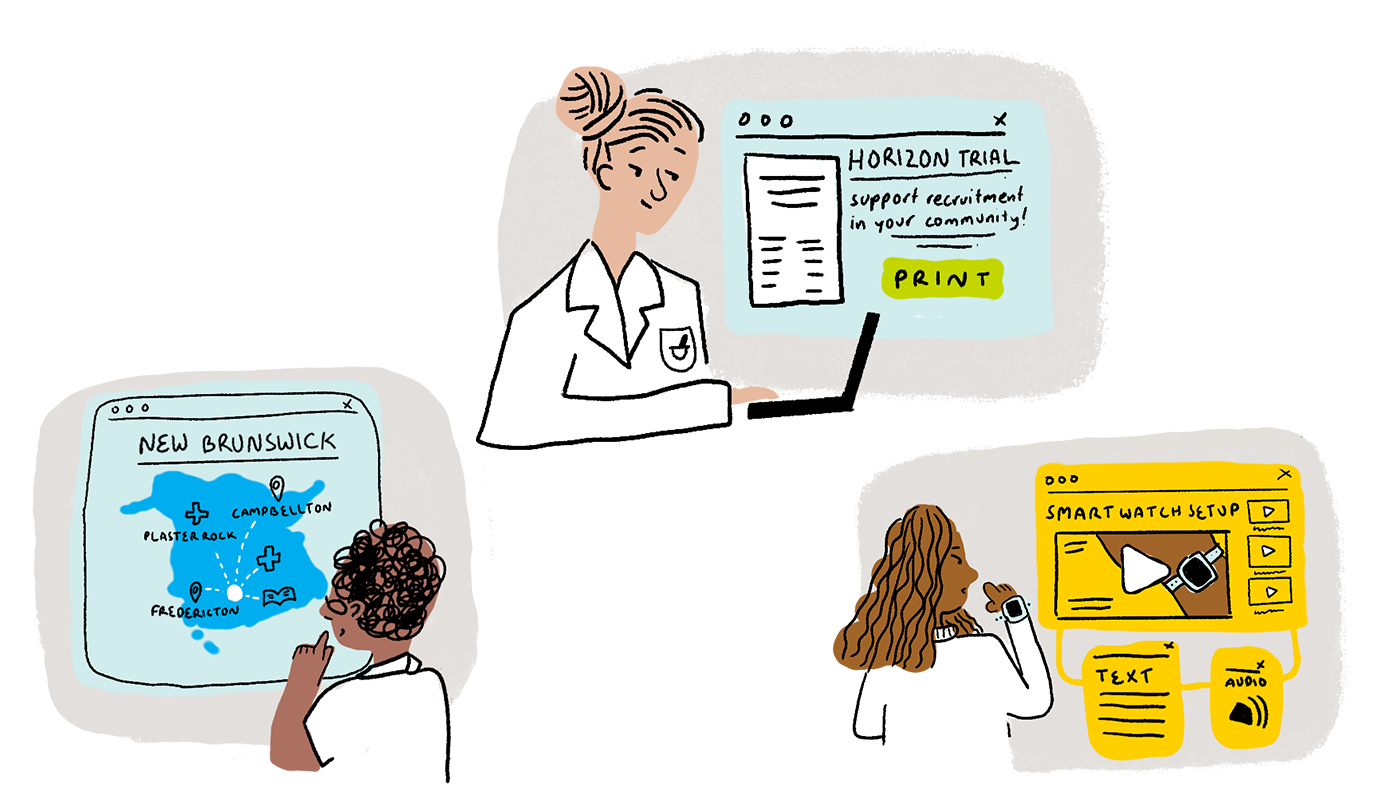
2. Key Platform Features + Experience Snapshots
We identified seven high-priority platform functions that support a positive experience for researchers and participants. These features highlight methods to engage participants and researchers, integrate the broader healthcare ecosystem in New Brunswick, and coordinate resources outside of the typical research team. The Key Platform Features are brought to life in Experience Snapshots, which depict how key features should ‘look and feel’ for users.
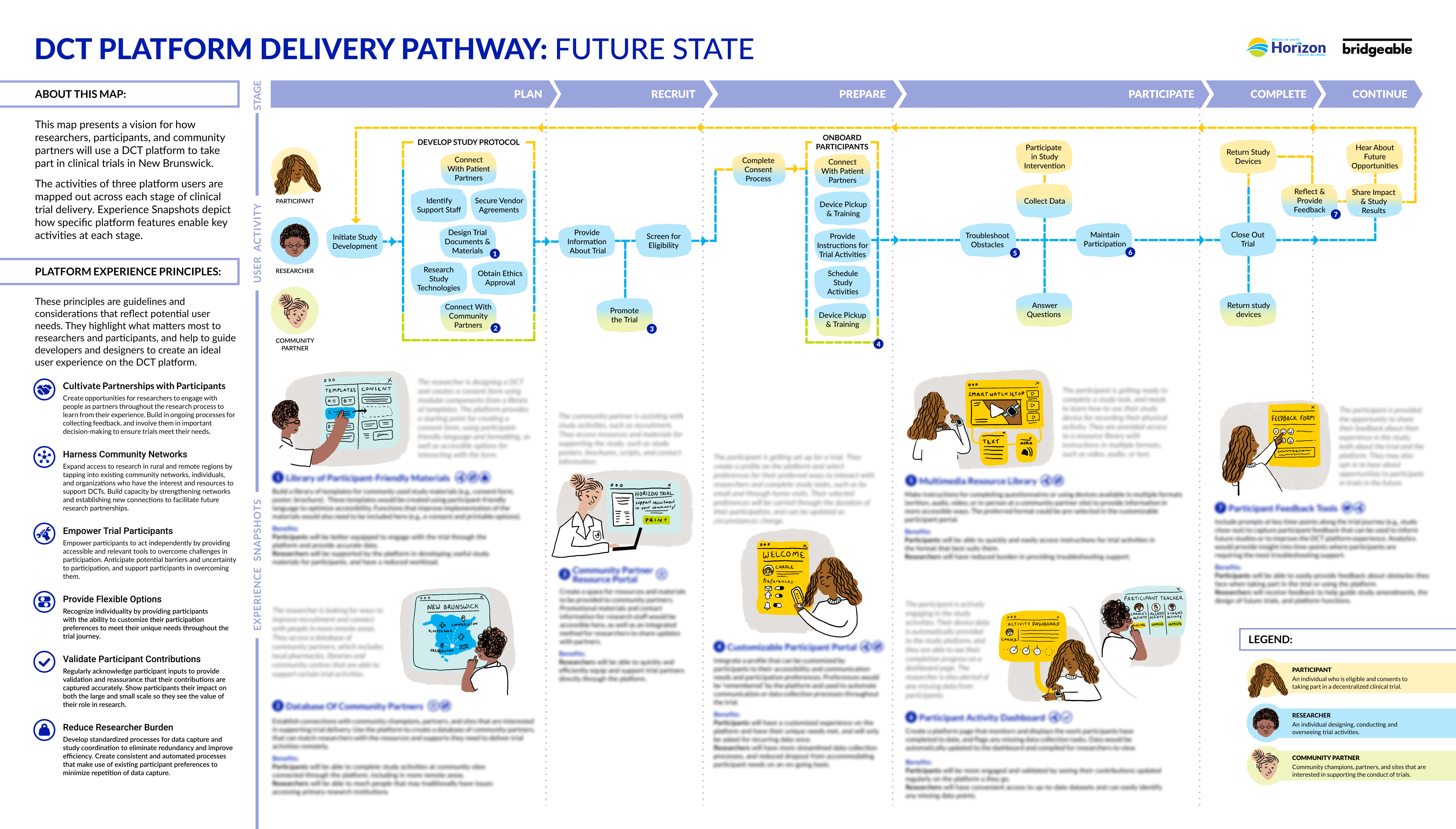
3. Future State Delivery Pathway
The Future State Delivery Pathway is a vision for how researchers, participants, and community partners will use a DCT platform to take part in clinical trials in New Brunswick. The activities of three platform users are mapped out across each stage of clinical trial delivery. Key Platform Features and Experience Snapshots depict how the DCT platform supports key activities. This pathway supports Horizon in communicating and socializing a vision for a future state DCT experience with external stakeholders.
Future directions
This project supports Horizon’s broader goal to modernize clinical trial delivery and ultimately improve healthcare access in New Brunswick. By moving forward with a deep understanding of the needs of participants, caregivers, and researchers, Horizon can position itself as a leader in DCT delivery. Providing DCTs can increase accessibility for participants and caregivers, and better equip researchers to conduct high-quality trials.
The outputs of this project support Horizon’s immediate next steps toward the development of a DCT platform that will increase patient representation and modernize clinical trials in New Brunswick.
References
1 — Office of the Premier. Pharmacists Now Treating Thirteen Common Ailments and Renewing Prescriptions for Most Medications. Ontario Newsroom. 2023 Jan 11. https://news.ontario.ca/en/release/1002633/pharmacists-now-treating-thirteen-common-ailments-and-renewing-prescriptions-for-most-medications
2 —Pharmacists can assess and prescribe for more illnesses and conditions. Government of New Brunswick. 2023 May 15. https://www2.gnb.ca/content/gnb/en/news/news_release.2023.05.0246.html