
Ideas by Bridgeable
The time is now to design equitable access to CAR T-cell therapies
Author
- Bridgeable
The promise of CAR T-cell therapies
Chimeric antigen receptor T-cell (CAR T) therapies are a new type of cancer treatment in which the patient’s own immune cells (T cells) are genetically modified in the laboratory to identify and eliminate cancer cells. These innovative therapies have brought hope to many patients who had almost no treatment options after their cancer returned multiple times.
Surgery, chemotherapy, and radiation have been the most common options to treat cancer over the past century. But in 2017, the era of “living drugs” began after the FDA approved the first CAR T-cell therapy1. So far, these therapies have been mostly used for patients whose cancer has returned several times despite receiving multiple treatments, but ongoing research suggests that they can also benefit patients in earlier stages of their treatment2.
Another immense benefit of CAR T-cell therapies is that when compared to other common cancer therapies, they usually require only a single or only a few infusions with a longer-term efficacy. Last year, researchers reported the case of two patients who, after receiving an infusion of their own modified immune cells, have been living without cancer for more than ten years3.
One example of how CAR T is transforming cancer care is multiple myeloma (MM), a type of blood cancer that commonly appears in later stages of life and is frequently diagnosed around the age of 70. Unfortunately, despite all the treatments available, MM is still considered incurable4. As a result, people living with MM usually undergo multiple treatment failures, many excruciating side effects, and a journey characterized by deep uncertainty. However, the approval of two CAR T-cell therapies5 to treat MM in 2021 and 2022 has been a turning point for many patients. It has opened the possibility of living longer and healthier by receiving a single infusion of a CAR T-cell treatment.
Because of its promising results, the number of studies conducted on CAR T has been growing exponentially. In 2020, there were over 500 ongoing studies worldwide6, and last year alone, more than 180 studies have been registered at clinicaltrials.gov. When it comes to FDA approvals, six CAR T-cell therapies have been endorsed to be available in the market, and the list is expected to grow in the coming years. This is just the beginning of a long journey to these therapies hitting the market. While focusing on their efficacy and safety is important, it is also essential to find solutions that allow access for the majority of patients who can benefit from them.
Despite their novelty, CAR T-cell therapies face the risk of exacerbating disparities in cancer care
Bringing CAR T-cell therapies to the market is complex. They involve very intricate manufacturing and supply chain processes, challenging logistical and regulatory demands to administer them, and significant reimbursement constraints. This complexity translates into a number of challenges that patients must face to receive a CAR T-cell therapy. They are required to stay close to a certified treatment center that administers these therapies for several weeks, have a 24/7 caregiver, comprehensive health insurance, and undertake the burden of expenses that are not covered by their insurance.
Many patients are not in a position to navigate those challenges and benefit from these innovative therapies. In fact, two critical barriers to accessing CAR T in the US are time spent traveling from the person’s home to a CAR T-cell treatment center and the associated travel expenses. Patients with higher poverty rates are the ones who face longer travel times and higher travel-related costs7,8.
The ‘Matthew effect’9 gives us a glimpse into a likely current scenario where only a few people with pre-existing social advantages—such as higher socioeconomic status—are the ones who are more likely to benefit from CAR T-cell therapies. The Matthew effect suggests that people with prior social advantages are more likely to benefit from further advantages, whereas the opposite happens with underserved populations. For example, multiple myeloma is more prevalent and deadly among Black populations. Although the 5-year survival rate for patients has improved over the past 20 years (from 35% to 50%), Black people have benefited least from that improvement10. In addition, even though studies indicate that Black people living with MM have better chances to respond positively to new therapies, the barriers they face to accessing treatments have restricted them from realizing the possibility of living longer and healthier lives. In this instance, health inequity–caused by systemic racism–appears to be one of the main determinants of the negative outcomes in Black populations living with MM. These inequities imply a big risk that the negative health outcomes might repeat or worsen with novel and complex-to-access therapies like CAR T.
Currently, companies are largely focused on tackling operational challenges in the CAR T space and identifying the necessary logistics, processes, and resources to make CAR T available to patients beyond clinical trial settings while complying with regulatory requirements (Figure 1). However, this presents a risk of overlooking critical aspects of the patient experience, like health equity and access. But based on our work with innovative cancer treatments, we believe there is a viable path to overcome this risk: using service design to embed health equity into the current operational focus in the commercialization of CAR T (Figure 2).
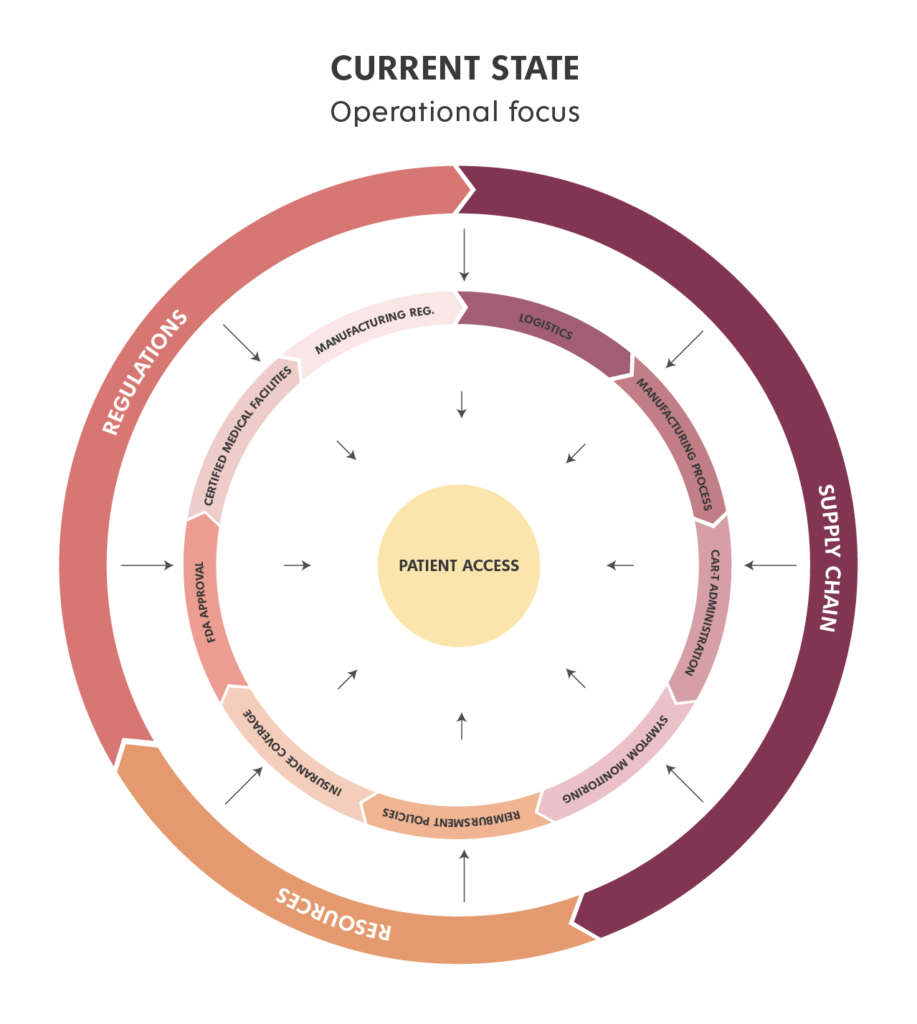
Figure 1
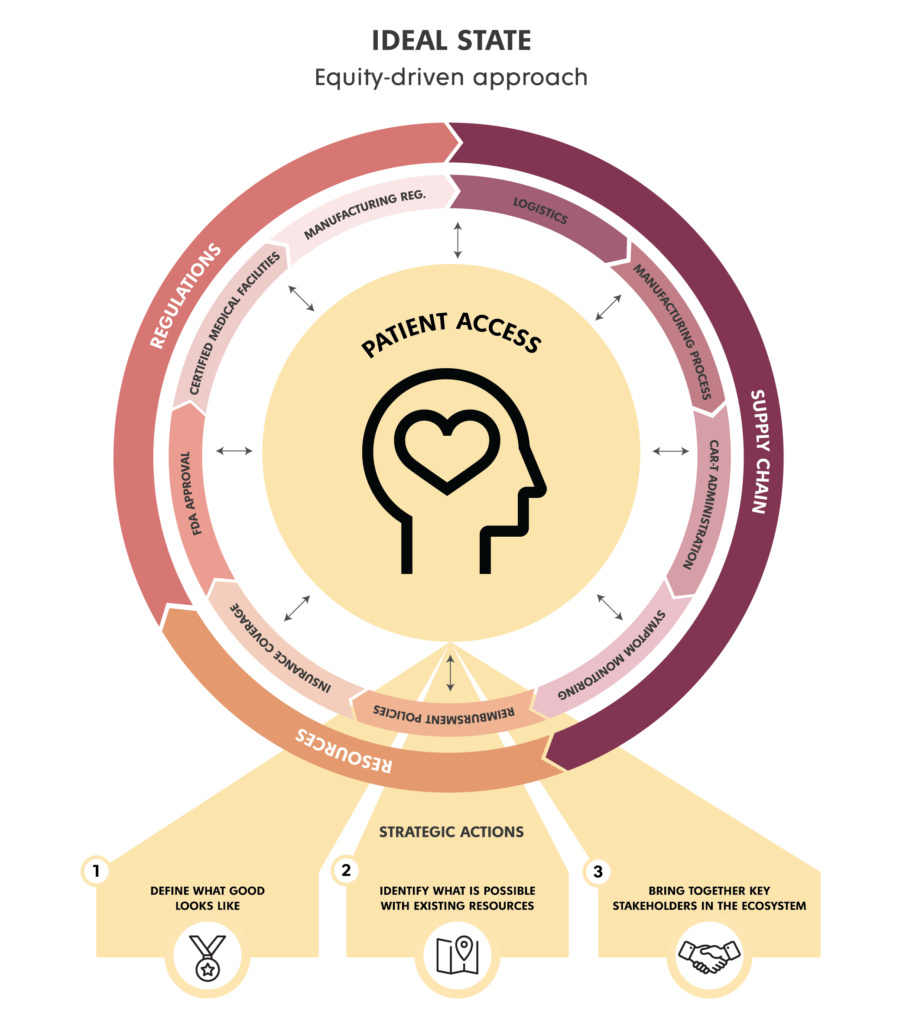
Figure 2
How might we embed health equity into the commercialization of CAR T?
An equity-driven approach uses service design to proactively create the necessary mechanisms to expand the benefits of CAR T-cell therapies and reach the highest number of people from multiple racial and socioeconomic backgrounds (Figure 2). This approach also addresses current health equity demands in our society and industry standards by meeting the needs of patients and their care partners, preventing barriers to care, and enabling culturally competent services that avoid rooted biases and discriminatory practices.
We acknowledge that this is not a simple undertaking as it involves a systemic transformation and coordinated effort. But we also see that now is the perfect moment to expand access to these groundbreaking therapies and design a compelling future state to work towards.
Changes to latent systems are possible with small, strategic actions that allow momentum to build towards a large-scale goal. Over the past two decades, we have learned there are three service design strategic actions that are crucial when designing for health equity and access. These actions represent a conversation starter and a roadmap to inspire action toward equitable access:
- Defining what good looks like
- Identifying what is possible to achieve within existing resources
- Bringing together key stakeholders in the ecosystem
Defining what good looks like
The first step is to understand the current state and collaboratively imagine the ideal state, which is built with input from internal stakeholders, key opinion leaders, patients, and their care partners. This strategic action is about defining our north star and the guiding principles that will drive all actions and strategies to achieve equitable access. Using a strategic foresight approach, we can build clarity around a range of possible future scenarios in order to develop resilient strategies. We apply a service design lens to this approach to go a step further and define how to implement those strategies and how those scenarios unfold from the perspective of people that inform the future and are impacted by it.
Identifying what is possible with existing resources
Once we understand our north star and guiding principles, we identify the resources and conditions needed to improve equitable access, the constraints involved, and the existing resources, enablers, and strategies needed for implementation. Using service blueprints, we can visualize desired future scenarios and lay the groundwork for implementation. This operational tool reflects how people, touchpoints, processes, resources, and technology are integrated to deliver a service. This tool not only helps orchestrate the necessary resources to improve equitable access but also enables stakeholder alignment and engagement for implementation.
Bringing together key stakeholders in the ecosystem
After identifying the resources needed to improve equitable access, the next step is developing mechanisms for genuine and synergistic collaboration across all stakeholders, from healthcare providers to patients, their care partners, pharmaceutical companies, community-based organizations, and beyond. Applying approaches like a community of practice and community engagement models is key to enabling the development of principles for strong collaboration. These models aim to achieve meaningful, ongoing relationships that strengthen a shared vision toward equitable access. At Bridgeable, we amplify the impact of both models by developing toolkits that curate and scale up learnings from the ecosystem.
Improving equitable access to novel therapies like CAR T-cell therapy involves a change in the entire ecosystem that requires intentionally designing for equitable access and actively bringing together the stakeholders in the ecosystem. The three strategic actions above represent a conversation starter. They intend to lay the roadmap to take these revolutionary therapies to the next level by amplifying their impact through health equity.
References
1 —Mullard, A. FDA approves first CAR T therapy. Nat Rev Drug Discov 16, 669. 2017 September 29. https://doi.org/10.1038/nrd.2017.196
2 —Whitefield, K. Bristol Myers Squibb and 2seventy bio Announce Topline Results from KarMMa-3 Trial Showing Abecma. Business Wire. 2022 August 10. https://www.businesswire.com/news/home/20220810005229/en
3 —Melenhorst, J.J., Chen, G.M., Wang, M. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509. 2022 February 02. https://doi.org/10.1038/s41586-021-04390-6
4 —Multiple Myeloma: Types of Treatment. American Society of Clinical Oncology. 2021 August. https://www.cancer.net/cancer-types/multiple-myeloma/types-treatment
5 —Current FDA-Approved Medications. International Myeloma Foundation. 2021 October 22. https://www.myeloma.org/multiple-myeloma-drugs
6 —Albinger, N., Hartmann, J. & Ullrich, E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther 28, 513–527. 2021 March 22. https://doi.org/10.1038/s41434-021-00246-w
7 —Snyder S, Chung KC, Jun MP, Gitlin M. Access to Chimeric Antigen Receptor T Cell Therapy for Diffuse Large B Cell Lymphoma. Adv Ther. 2021 July 23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8408091/
8 —Snyder S, Albertson T, Garcia J, Gitlin M, Jun MP. Travel-Related Economic Burden of Chimeric Antigen Receptor T Cell Therapy Administration by Site of Care. Adv Ther. 2021 July 18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8342383/
9 —Bothner, M.S., Haynes, R., Lee, W. & Smith B.S. When do Matthew Effects Occur?. The Journal of Mathematical Sociology, 34:2, 80-114. 2010 March 22. https://www.tandfonline.com/doi/abs/10.1080/00222500903310960
10 —Marinac, C.R., Ghobrial, I.M., Birmann, B.M. et al. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 10, 19. 2020 February 17. https://doi.org/10.1038/s41408-020-0284-7