
Ideas by Bridgeable
Artificial intelligence and the future of patient experience
Author
- Bridgeable
The A.I. revolution and its caveats
The concept of Artificial Intelligence (A.I.) was first described in 1950, referring to the use of computers to simulate human intelligence. The A.I. field has developed exponentially over the past decades to the point that we now have complex algorithms that simulate several human brain functions. The use of A.I. has permeated almost all areas of our lives, from personal assistants to the personalized content we see on social media, fraud detection, diagnosis of diseases, and many other applications. Overall, A.I. is predicted to become increasingly ubiquitous in our lives.
Algorithms and data are two critical components of A.I. While algorithms are the instructions given to a computer to perform a specific task, data is the raw material fed into algorithms to identify patterns and relationships and make predictions or decisions. All components of A.I. are subject to systematic errors, also known as biases. Biases can be distortions of reality, such as extrapolating findings from non-representative samples that exclude specific populations in clinical trials. But they can also reflect or amplify social prejudices like racism or sexism. For instance, while some facial recognition software has failed to distinguish dark skin tones or female faces, other hiring algorithms have favored male candidates when recruiting. Although biases are one of the main challenges in the A.I. field, the key to ensuring a positive evolution of A.I. lies in providing accurate and timely feedback to A.I. systems.
A.I. in pharma
The pharmaceutical industry is another critical area that A.I. is transforming. Companies are increasingly partnering with A.I. providers to improve multiple processes in their value chain. For example, A.I. is now used to accelerate the discovery of new drugs, make the manufacturing processes more efficient, develop personalized treatments for patients, predict future outbreaks, optimize the design of clinical trials, and make a more personalized outreach to patients and providers for marketing and sales purposes. A.I. can bring significant benefits to all areas of the industry, including patients and providers. However, we must address several risks and challenges to realize the potential benefits of A.I., mainly when using it in areas that imply direct contact with patients or providers, such as clinical trial development and marketing and sales.
The pharmaceutical industry is heavily regulated, and any A.I.-based solution will need to comply with strict regulatory requirements. This can be a significant challenge for A.I., especially when it comes to the development of clinical trials and marketing materials. Specifically, companies seeking approval for late-stage clinical trials will soon be required by the U.S. Food and Drug Administration (FDA) to submit a plan to ensure participant diversity. While A.I. can accelerate several participant selection processes, we have learned that diversity requires genuine community engagement strategies and inspection mechanisms that assess the quality of A.I. outputs to ensure the highest standards when aiming for diversity and patient centricity. Another example is the development of marketing materials. They not only need to be compliant with regulations but also use inclusive, accessible language and visuals, and respond to the needs of patients and providers.
Ethical considerations are another regulatory requirement when designing and conducting clinical trials. One of the biggest challenges for A.I. is avoiding unfair and discriminatory biases that favor some groups over others. Pharmaceutical companies need to ensure that the A.I. models they use are developed and trained with ethical and human-centered considerations to prevent unfair discrimination against certain patient or provider groups.
Another important consideration when using A.I. in pharma is developing the mechanisms to effectively and timely assess the quality of A.I. outputs. Pharmaceutical organizations are often using A.I. products developed by external vendors. While this improves efficiency, they must ensure that these algorithms produce outputs compliant with regulations, meet industry standards, and are user-centered. An ideal scenario would be having patient communications built with patient and provider-centered considerations from the get-go, but it will take a substantial amount of time and a lot of feedback for A.I. to produce outputs that are regulatory-compliant and patient-centered.
The reality is that while less efficient, both marketing and sales materials and clinical trials made by humans are of higher quality. They achieve the necessary patient and provider-centricity that front-running pharmaceutical organizations require. Though, as A.I. continues to evolve (and quite rapidly), with the support of service design, it has the potential to streamline many of these processes and limit the need for human intervention in, at the very least, materials for both: clinical trials and marketing.
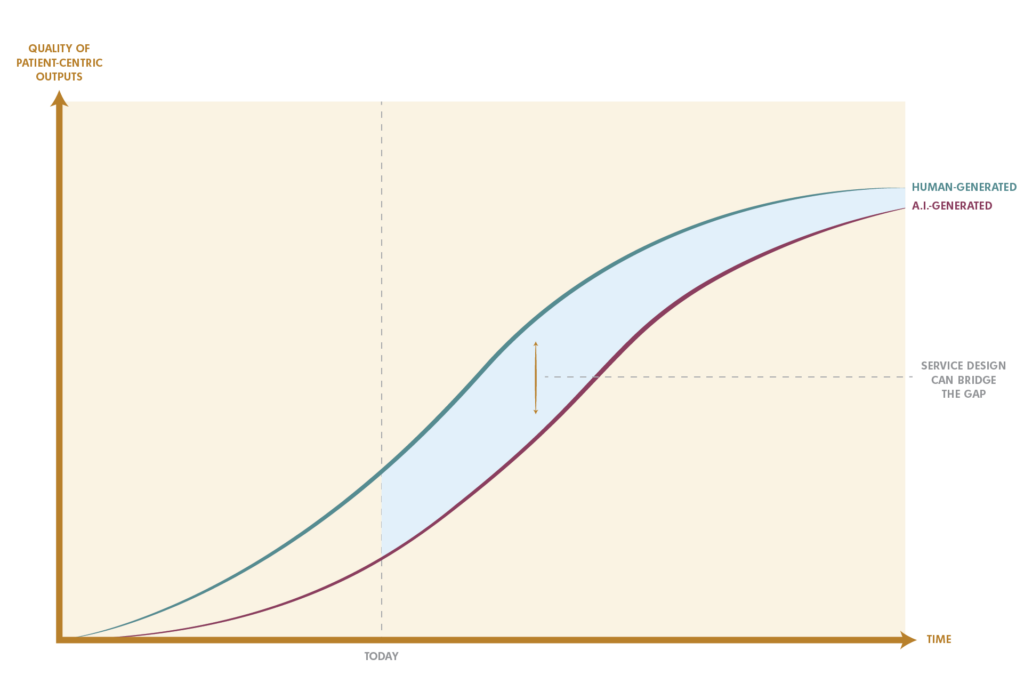
Bringing a human-centered lens to A.I. outputs
Leveraging service design can enable organizations to maximize the existing benefits of A.I. by bridging the gap between A.I. outputs and patient and provider needs. It can also support the wheels that are in motion to improve the quality of future outputs, reducing the demanding input and resources.
The first step is to build a set of best practices that enhance and amplify the benefits of using A.I. for outputs in the pharmaceutical landscape. Similarly, Bridgeable has worked with Bristol-Myers Squibb (BMS) on The Universal Patient Language (UPL), an organizational capability and set of resources that enables better patient-facing communications. The UPL is leveraged at all levels of BMS to deliver an enhanced patient experience across channels and therapeutic areas.
Bringing together a diverse set of stakeholders, including patients, caregivers, healthcare providers, advocacy organizations, and subject matter experts, allows us to reimagine and redesign how complex pharmaceutical topics should be communicated to patients and caregivers. This is the subject matter and language that bridges the gap between A.I.’s outputs and how they need to be communicated. Implementing this set of resources and best practices solves the practical challenges of building new patient communications.
Going beyond proactivity in implementing human-centered improvements to A.I.-generated outputs, service design can enable effective feedback to ensure pharmaceutical organizations can communicate:
- Which aspects of A.I.-generated outputs can be improved based on current standards and best practices;
- How they can improve, and;
- How to translate these needs to their A.I. vendors or internal A.I. systems.